Can Broncho-Vaxum reduce COVID-19 infection?
In a new study from the University of Arizona, researchers have found that a mixture of bacterial extracts known as OM-85, used in Europe to treat respiratory infections, may be a new way to prevent or reduce infection with SARS-CoV-2, the virus that causes COVID-19.
 |
| The University of Arizona |
OM-85 is a bacterial solution, a mixture of molecules derived from the cell walls of bacteria, marketed outside the United States as Broncho-Vaxom for the preventive treatment of respiratory infections in children and adults.
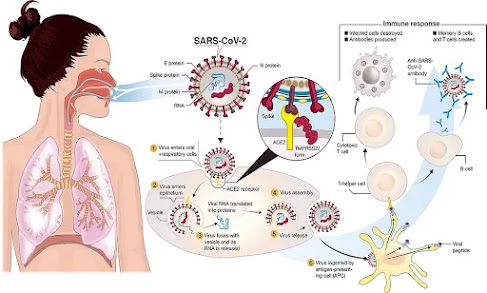
When SARS-CoV-2 enters the lungs, it binds to receptors, including the angiotensin-converting enzyme receptor 2 called ACE2, on the outer membranes of lung cells.
The cellular enzyme changes the shape of the virus protein, allowing SARS-CoV-2 to penetrate the membrane and infect the cell.
The researchers found that pretreatment of the cells with the bacterial extract OM-85 prevented infection by SARS-CoV-2, due to its effective ability to act on the ACE2 receptor.
The mechanism of action of OM-85 in fighting viral infections is different from that of vaccines since the efficacy of vaccines lies in their action on the viral protein.
OM-85 Broncho-Vaxom®, a bacterial lysate, reduces SARS-CoV-2 binding proteins in human bronchial epithelial cells.
In clinical studies, OM-85 Broncho-Vaxom®, a bacterial lysate, reduced viral respiratory infections. Infection of epithelial cells by SARS-CoV-2 depends on the interaction between its spike protein (S protein) and host cell membrane proteins.
In this study, we investigated the effect of OM-85 on the expression of S-protein binding proteins in human bronchial epithelial cells.
Human bronchial epithelial cells were treated with OM-85 for 5 days. Expression of SARS-CoV-2 receptor, angiotensin-converting enzyme 2 (ACE2), transmembrane protease subtype 2 (TMPRSS2), dipeptidyl peptidase-4 (DPP4), and a disintegrin and metalloprotease 17 (ADAM17) was determined by western blotting and quantitative RT-PCR. ACE2 (s)soluble, heparan sulfate, heparanase, and hyaluronic acid were determined by ELISA.
OM-85 significantly reduced the expression of ACE2 (p < 0.001), TMPRSS2 (p < 0.001), DPP4 (p < 0.005), and cellular heparan sulfate (p < 0.01), whereas ADAM17 expression (p < 0.02) was significantly increased. In addition, OM-85 increased the levels of sACE2 (p < 0.05), hyaluronic acid (p < 0.002), and hyaluronan synthase 1 (p < 0.01).
Thus, infection of a pseudotypic lentivirus with the SARS-CoV-2 spike protein was reduced in cells pretreated with OM-85.
These results suggest that OM-85 can reduce the binding of SARS-CoV-2 S protein to epithelial cells by modifying host cell membrane proteins and specific glycosaminoglycans. Therefore, OM-85 can be considered an adjuvant to COVID-19 treatment.
Conflict of interest statement
The funders had no influence on the design of the study, the collection, analysis, or interpretation of the data, the preparation of the manuscript, or the decision to publish the results.
OM-85 bacterial lysate inhibits SARS-CoV-2 infection of epithelial cells by downregulating SARS-CoV-2 receptor expression.
BACKGROUND: There is an urgent need for treatments for the 2019 severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), but therapeutic options remain limited.
SARS-CoV-2 infects cells through interactions between its spike protein (S protein) and angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) in host cells.
Several cells and organs are targeted, including airway epithelial cells. OM-85, a standardized lysate of human respiratory bacteria with potent immunomodulatory properties and an impeccable safety profile, is widely used to prevent recurrent respiratory infections.
We found that administration of OM-85 into the airways inhibited Ace2 and Tmprss2 transcription in mouse lungs, suggesting that OM-85 may prevent SARS-CoV-2 interactions with host cells.
Aims: We investigate whether and how OM-85 treatment protects non-human and human primate epithelial cells from SARS-CoV-2.
Methods: We measured ACE2 and TMPRSS2 mRNA and protein expression, cellular binding of SARS-CoV-2-S1 protein, formation of SARS-CoV-2-S1 pseudotyped lentiviral particles, and infection of SARS-CoV-2 cells in kidney, lung, and intestinal cell lines, primary human bronchial epithelial cells, and in HEK293T cells transfected with ACE2 and treated with OM-85 in vitro.
RESULTS: OM-85 significantly increased ACE2 and TMPRSS2 transcription and ACE2 protein expression on the surface of epithelial cell lines and primary bronchial epithelial cells.
OM-85 also strongly inhibited the binding of SARS-CoV-2-S1 to SARS-CoV-2-S protein, the entry of pseudotypic lentivirus into epithelial cells, and the infection of epithelial cells by SARS-CoV-2.
These effects of OM-85 appear to be dependent on the down-regulation of the SARS-CoV-2 receptor.
Conclusions: OM-85 inhibits SARS-CoV-2 infection of epithelial cells in vitro by down-regulating SARS-CoV-2 receptor expression. Further studies are needed to determine whether OM-85 can prevent and/or reduce the severity of coronavirus disease in 2019.
SARS-CoV-2 and SARS-CoV Spike-mediated cell fusion differ in their receptor expression and proteolytic activation requirements.
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infects cells through the interaction of its spike protein (SARS2-S) with angiotensin-converting enzyme 2 (ACE2) and the activation of proteases, particularly transmembrane serine protease 2 (TMPRSS2).
Viruses can also spread by the fusion of infected cells with uninfected cells. We compared CEA2 expression conditions, proteolytic activation, and sensitivity to inhibitors of SARS2-S and SARS-CoV-S (SARS1-S)-mediated cell fusion.
SARS2-S-induced fusion was moderately enhanced by TMPRSS2 and strongly enhanced by CEA2, whereas SARS1-S-induced fusion was strongly enhanced by TMPRSS2 and less strongly enhanced by CEA2 expression.
In contrast to SARS1-S, SARS2-S-mediated cell fusion was efficiently activated by batimastat-sensitive metalloproteases. Mutation of the S1/S2 proteolytic cleavage site reduced effector-target cell fusion when CEA2 or TMPRSS2 was limiting and made SARS2-S-induced cell-cell fusion more dependent on TMPRSS2.
When CEA2 and TMPRSS2 were abundant, the initial target cell-carrier fusion was not altered compared with wild-type (wt) SARS2-S, but the syncytia remained smaller.
The S2 cleavage site mutation (S2') specifically abrogates TMPRSS2 activation for cell fusion and SARS2-S-induced pseudoparticle entry, but still allows activation of metalloproteases for cell fusion and cathepsins for particle entry.
Finally, we found that the TMPRSS2 inhibitor bromhexine, unlike the Camostat inhibitor, cannot reduce TMPRSS2-activated cell fusion in SARS1-S and SARS2-S.
Paradoxically, bromhexine increased cell fusion in the presence of TMPRSS2, whereas its metabolite, ambroxol, showed inhibitory activity under certain conditions.
In Call-3 lung cells, ambroxol weakly inhibited SARS2-S-induced lentiviral pseudoparticle entry, and both drugs showed a dose-dependent trend toward weak inhibition of authentic SARS-CoV-2.IMPORTANT Cell fusion allows viruses to infect neighboring cells without having to produce free virus and contributes to tissue damage by generating virus-infected syncytia.
Our results show that the S2 cleavage site is essential for TMPRSS2 activation and reveal important differences between SARS-CoV and SARS-CoV-2, including a greater dependence of SARS-CoV-2 on CEA2 expression and metalloproteinase activation for cell fusion.
Brominexin, which is believed to be a TMPRSS2 inhibitor, is currently in clinical trials against coronavirus 2019 disease. Our results suggest that bromhexine potentiates fusion under certain conditions.
Therefore, we caution against the use of bromhexine at high doses until its effects on SARS-CoV-2 spike activation are better understood.
The related compound ambroxol, which, like bromhexine, is used clinically as an expectorant, showed no activating effect on cell fusion.
Both compounds showed weak inhibitory activity against SARS-CoV-2 infection at high concentrations, which may be clinically feasible for ambroxol.



Post a Comment